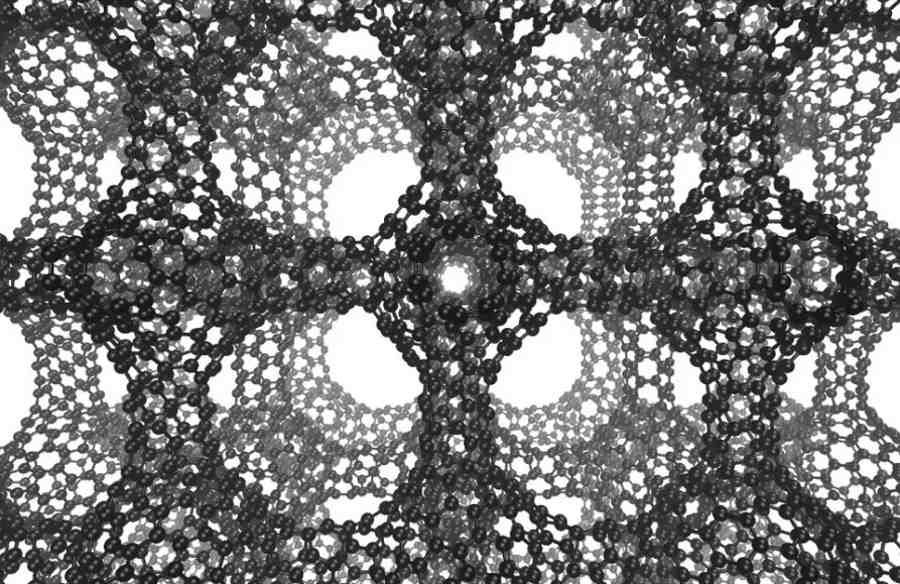
Long sought-after carbon structure created by accident
Carbon is a very interesting element, and pure carbon structures have many uses and unique properties. It is wonderfully roFG – from diamondoIn showing exceptional hardness to graphite, with ktoWe are in contact with them, for example, by using leadowka. Diamond AND graphite are trojdimensional carbon structures, but carbon atoms can roalso create two-dimensional structures. Graphene – a flat sheet of atomow coal, whichory is not only the strongest material on Earth, but has roAlso high electrical conductivity – is such a structure. One should also not forget about carbon nanotubes or fullerenes. Now the already numerous family has been joined by schwarzite.
Chemists at the University of California, Berkeley, have demonstrated that three carbon structures recently created by scientistsoin South Korea and Japan are in fact long sought-after schwarzites, whichohe existence of which had been predicted for a long time. A paper on the subject appeared in „Proceedings of the National Academy of Science”.
The new structures were built inside the zeoliteow, crystalline forms of silicon dioxide, which theore more commonly used as water softeners in laundry detergents or in the production of fertilizersoIn mineral or gasoline. A vapor containing carbon molecules was injected into the zeolite, allowing the carbon to cluster into a two-dimensional graphene-like sheet surrounding the walls of the poreoin the mineral. Through stress, the surfaces achieve a negative curvature, like a saddle. Then just dissolve the zeolite and we have schwarzite.
Scientists from Japan and South Korea were unaware of their discovery, over which theorym chemists have been working on for decades. The Schwarzites created by accident while studying the properties of the so-called “silicon dioxide”. carbonoin zeolite-templated carbons (ZTCs). No one on the work of Asian scientistsow did not pay attention, dopoki it was not dealt with by chemists from the US.
Schwarzit was identified by Efrem Braun with his colleagues at UC Berkeley. The researchers did this on the basis of their negative curvature. By the way, they also developed a wayob for predicting whoore zeolites will be suitable for the formation of schwarzite.
– We now have a recipe for how to make these structures, which is important because if we can make them, we can also study their properties – Said Berend Smit, an expert in materialsoIn porous I metal-organic structures at UC Berkeley.
Based on theoretical work, chemists predict that schwarzites will have unique electronic, magnetic and optical properties, due to whichorym will be useful as supercapacitors, battery electrodes and catalysts.
In the 1880s, German physicist Hermann Schwarz studied negatively curved structures that resemble bubble surfacesoin soapstone, and when theoretical work on carbon cage particles gained momentum in the 1990s. his name was connected to hypothetically curved carbon sheets. Hence the name of the material.
Discovery of fluereneow and graphite were honored with Nobel prizes in 1996 and 2010, respectively. The researchers behind the schwarzite are also quietly probably hoping for fame I Nobel.
Sourceobackground: University of California – Berkeley, fot. Yongjin Lee/ Efrem Braun, UC Berkeley


